Alcohol consumption can indirectly cause damage to our DNA and elevate cancer risk. KJ Patel’s group in the MRC Laboratory of Molecular Biology’s (LMB’s) Protein and Nucleic Acid Chemistry (PNAC) Division, in a close collaboration with Puck Knipscheer’s group at the Hubrecht Institute in Utrecht, has identified a novel repair mechanism for DNA damage created by by-products of alcohol metabolism. Professor Patel will be moving to take up the Directorship of the MRC Weatherall Institute of Molecular Medicine (MRC WIMM) and the MRC Molecular Haematology Unit (MRC MHU) as announced last week.
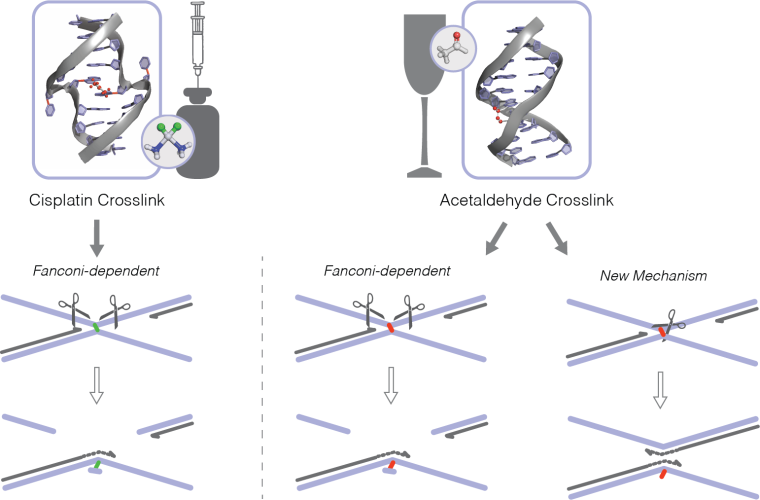
DNA crosslinks are a very toxic form of DNA damage that block a cell’s ability to copy its DNA or transcribe it in order to produce proteins. This type of DNA damage is known to be caused by cisplatin, an important chemotherapy drug, and aldehydes, including acetaldehyde, a by-product of alcohol breakdown in our bodies. Professor Patel’s group had previously found that the Fanconi Anaemia (FA) DNA repair pathway fixes the DNA crosslinks formed by aldehydes.
In this study, the team wanted to investigate how the rules for repair differ between acetaldehyde-derived and cisplatin-derived crosslinks. To do this, Michael Hodskinson and his colleagues in Professor Patel’s group worked with Jason Chin’s group, also in the LMB’s PNAC Division, to devise a chemical method to make a single acetaldehyde crosslink in a short circular DNA molecule. Although these crosslinks could form in just a few seconds in a cell, it took the better part of 3 years to make them in a test tube.
Collaborating with Puck Knipscheer, who is an expert in creating cell-free DNA copying extracts, the team then incubated the synthetic circular DNA in frog egg extracts that have been primed to activate DNA copying and was able to study how a single crosslink was repaired. As expected, they saw rapid activation of the FA DNA repair pathway, leading to cutting out of the crosslink. However, they also made a surprising discovery.
A significant number of acetaldehyde crosslinks were repaired by a new pathway that did not need to excise the DNA crosslink and did not create a break in the DNA strand. More detailed experiments suggested that this new repair mechanism breaks the crosslink from within the DNA helix. The new pathway, however, cannot fix DNA crosslinks made by cisplatin, which must be repaired by the FA pathway. “
Perhaps the sole reliance on the FA DNA repair pathway for crosslinks caused by chemotherapeutic drugs might explain why they can so efficiently kill cancer cells - Professor Patel
Future work aims to determine the factors that carry out this new DNA repair pathway, and to determine its physiological importance. “This new research underscores that DNA crosslink repair, which is vital for our wellbeing, is only just beginning to be understood,” says Michael.
The work was funded by the MRC, Dutch Cancer Society, Netherlands Organisation for Scientific Research, Uehara Memorial Foundation, Mochida Memorial Foundation for Medical and Pharmaceutical Research, Wellcome Trust, Cancer Research UK, the European Research Council and a Japan Society for the Promotion of Science Postdoctoral Fellowship for Research Abroad.
Read the full paper.
Adapted from article originally published on the MRC LMB website.

 ©
MRC Laboratory of Molecular Biology
©
MRC Laboratory of Molecular Biology